In the ever-evolving landscape of clinical research, staying up to date with regulatory guidelines is crucial for ensuring patient safety and data integrity. The recent release of the International Council for Harmonisation’s (ICH) Good Clinical Practice (GCP) E6 (R3) guideline marks a significant milestone in the field. This third revision of the internationally recognized standard is set to reshape the entire clinical trial lifecycle, from design and conduct to monitoring, recording, and reporting.
As we approach the implementation date of July 23, 2025, it’s essential for all stakeholders in the clinical research industry to understand the key changes and their implications. This blog post aims to provide a comprehensive overview of ICH GCP E6(R3), highlighting its importance and the ways in which it will transform clinical trials.
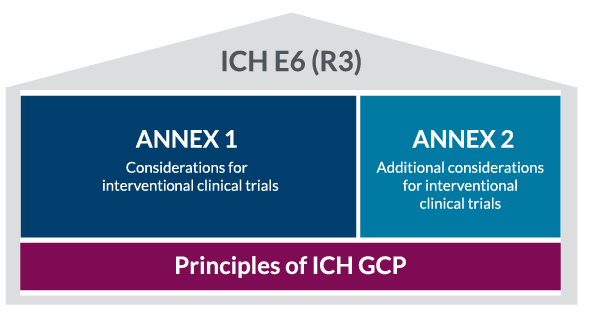
A New Structure for a New Era
One of the most notable changes in E6 (R3) is its restructured format. The guideline now comprises three main components: a core section outlining general principles, followed by two annexes. Annex 1 focuses on interventional trials, while Annex 2 addresses non-traditional interventional trials. This new organization provides a more comprehensive and adaptable framework, acknowledging the varied landscape of modern clinical research.
Embracing Technological Advancements
The E6(R3) guideline recognizes the increasing role of technology in clinical trials. It provides expanded guidance on the use of electronic systems, including digital health technologies (DHTs) and electronic sources (eSources). This acknowledgment of technological progress extends to the informed consent process, with more detailed guidance on using technologies to inform participants and obtain their consent.
Furthermore, the guideline adopts a “media neutral” approach, ensuring that GCP principles apply consistently across all platforms, whether traditional or digital. This forward-thinking stance prepares the industry for future technological innovations while maintaining high standards of data integrity and patient safety.
Quality by Design: A Fit-for-Purpose Approach
E6 (R3) introduces a “fit for purpose” approach to quality-by-design, emphasizing that the quality of a clinical trial should be tailored to meet its specific objectives. This involves identifying critical-to-quality factors and designing protocols and processes that ensure the reliability and integrity of trial results. By doing so, the guideline encourages a more thoughtful and efficient approach to trial design and execution.
Enhancing Patient-Centricity
A key focus of E6 (R3) is the increased emphasis on study participants. The guideline highlights the importance of considering participants’ perspectives and experiences in trial design and conduct. This patient-centric approach aims to minimize participant burden, enhance the overall trial experience, and potentially improve recruitment and retention rates.
The recognition of Decentralized Clinical Trials (DCTs) in the guideline further underscores this patient-centric shift. DCTs, which utilize digital technologies to conduct various trial activities remotely, can enhance patient convenience and participation while reducing the burden on clinical sites.
Strengthening Quality Management Systems
The new revision places a stronger focus on implementing robust Quality Management Systems (QMSs) to maintain and enhance the quality of clinical trials. This includes proactive risk management, continuous improvement processes, and ensuring compliance with regulatory requirements. By emphasizing QMSs, the guideline aims to create a culture of quality throughout the entire clinical trial process.
Recognizing the Role of CROs
The new guideline acknowledges the various roles that Contract Research Organizations (CROs) play in clinical trials. It provides detailed guidance on the responsibilities and expectations for CROs, ensuring that their contributions are well-integrated into the overall trial management and quality assurance processes. This recognition reflects the evolving landscape of clinical research and the increasing importance of partnerships in conducting successful trials.
Preparing for Implementation
As the industry prepares for the implementation of E6 (R3), organizations are taking proactive steps to ensure compliance. Parexel Academy has initiated a comprehensive preparation process. This includes reviewing and assessing the new guidelines, updating processes and controlled documents, revising training materials, and creating centralized resources for information and updates.
Such preparation is crucial for a smooth transition to the new guidelines. It involves not only understanding the changes but also adapting existing systems and processes to align with the new requirements. Parexel Academy is already in the process of updating all current training materials with the new guidelines to ensure all learners are equipped with the most up-to-date knowledge.
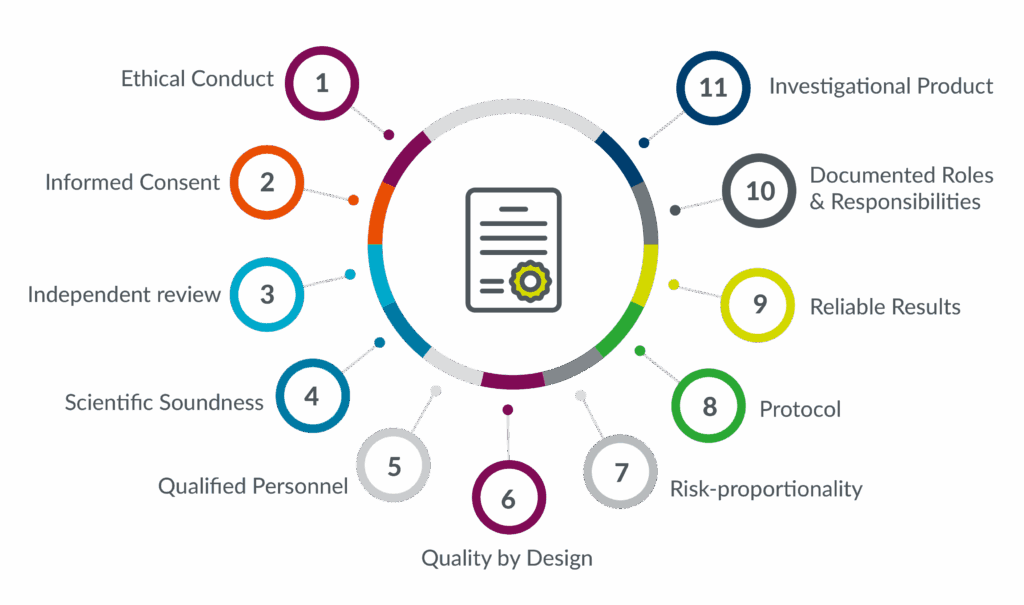
The Road Ahead
The implementation of ICH GCP E6 (R3) represents a significant advancement in the conduct of clinical trials. By incorporating flexibility, patient-centricity, and lessons from recent public health emergencies, the new guidelines aim to enhance the quality, efficiency, and reliability of clinical trials.
As we move towards the implementation date, it’s crucial for all stakeholders in the clinical research industry to familiarize themselves with these changes. This includes sponsors, investigators, CROs, regulatory bodies, and ethics committees. By understanding and embracing these new guidelines, we can collectively work towards more effective, efficient, and patient-centered clinical trials.
The journey towards implementing E6(R3) is not just about compliance; it’s an opportunity to elevate the standards of clinical research. It challenges us to think more critically about trial design, to leverage technology more effectively, and to keep the patient at the center of everything we do.
As we enter this new era of clinical trials, Parexel Academy embraces the spirit of innovation and continuous improvement embodied in ICH GCP E6(R3). By doing so, we can ensure that clinical research continues to advance, ultimately leading to better outcomes for patients and more efficient development of life-saving treatments.
If you want to learn more about the newest ICH GCP revision, take a look at Parexel Academy’s new Virtual Postgraduate Certificate Program which covers all of the changes and more!


